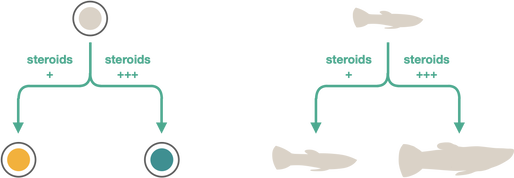
Steroid control of cell fate and organismal phenotype
Tissue- and cell-specific steroid signaling
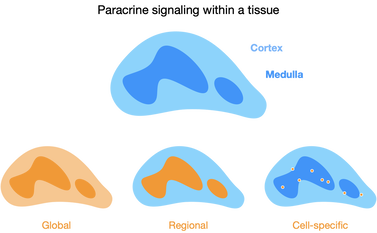
Glucocorticoids from the adrenal glands and circulate throughout the body, diffusing through tissues to coordinate systemic responses. However, certain tissues, including the thymus, can independently synthesize their own glucocorticoids. Thymic epithelial cell-synthesized glucocorticoids signal developing T cells and are necessary for generation of a competent adaptive immune system.
We are exploring how paracrine glucocorticoid signaling occurs in the thymus. Rather than widespread diffusion, thymus glucocorticoids may be targeted to particular T cells in the thymus, a novel mechanism of glucocorticoid signaling.
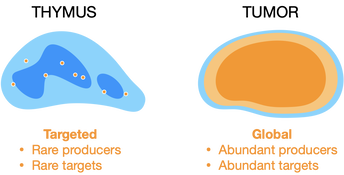
Local steroid production differs across contexts. In the thymus, medullary epithelial cells independently synthesize glucocorticoids, and these appear to be delivered to a specific subset of developing T cells. This suggests that glucocorticoids can act as cell-targeted signals. In tumors, cancer cells amplify blood-borne glucocorticoids to create an immunosuppressive microenvironment, preventing CD8+ T cells from killing cancer cells.
We are exploring how local steroids promote or inhibit immune responses, and how these can be specifically targeted to regulate immunity in pathological conditions.
Steroid regulation of cell development & differentiation
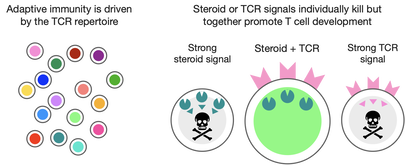
T cells recognize pathogens or cancer cells via the T cell antigen receptor (TCR). Each T cell expresses a unique TCR, and the huge diversity of TCRs on different T cells allows recognition of almost any antigen. This diversity is generated by random recombination of TCR genes during T cell development in the thymus. T cells with nonfunctional TCRs are eliminated (as these aren't useful), as are T cells with strongly self-reactive TCRs which might cause autoimmunity (negative selection). Only thymocytes with with moderately signaling TCRs become mature T cells. Steroids are potently immunosuppressive and can kill T cells. However, steroids antagonize TCR signaling in the thymus, allowing survival of T cells that would otherwise be negatively selected. In this way, steroids strengthen the TCR repertoire.
We are studying the T cell signaling events that lead to different selection outcomes, and exploring how TCR and steroid signaling networks interact to promote survival.
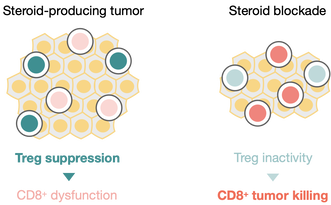
Tumors have multiple ways of evading immune responses, in particular producing molecules to suppress cancer-killing T cells. We have found that multiple tumor types amplify circulating glucocorticoids to create a potently immunosuppressive environment. In addition to suppressing the activity of killer T cells, these also activate regulatory T cells, which function as potent suppressors of immunity.
We are studying how steroids control regulatory T cell phenotypes, and act within the tumor to potentiate their immunosuppressive actions. We are also studying how tumor-specific ablation of steroid production alters regulatory T cells to take on alternative phenotypes and reduces tumor progression. In combination with immunotherapy, inhibition of glucocorticoids appears to additionally help control tumor progression.
We are studying how steroids control regulatory T cell phenotypes, and act within the tumor to potentiate their immunosuppressive actions. We are also studying how tumor-specific ablation of steroid production alters regulatory T cells to take on alternative phenotypes and reduces tumor progression. In combination with immunotherapy, inhibition of glucocorticoids appears to additionally help control tumor progression.
Detection and quantification of cell-specific steroid signaling
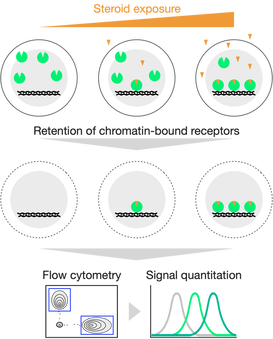
Steroid receptors and other nuclear receptors are ligand-dependent transcription factors: their activity is rapidly turned on and off by ligand presence/absence. Nuclear receptors drive cell development, differentiation, and death, and are important in various diseases. However, current techniques cannot quantify cell-specific ligand exposure. We have developed a technique to use endogenous receptors as biosensors, for the first time identifying and quantifying signals within single cells.
Currently, we are using this technique as an unbiased assay of tumor-produced steroids, as it detects any receptor-activating ligands. We are also developing tools identify steroid-signaled cells in tissue sections and in live cells.